While many people in Germany continue to wait for a vaccination appointment and a consequential improvement in the risk situation posed by COVID-19, the importance of production and logistics processes in the pharmaceutical industry is repeatedly made evident time and time again. Production facilities for vaccines are being newly built, distribution volumes closely monitored and transports planned.
Throughout transport, storage and handling, processes in the pharmaceutical sector must comply with complex regulations and are subject to strict documentation requirements. And for good reason, because the products are often critical to handle in terms of temperature, for example, and are often intended to save nothing less than lives. At the same time, the logistical processes must be as efficient and stable as possible.
Pharmaceutical goods impose high demands on warehouse logistics
These regulated processes and the continuous tracking of, for example, inbound/outbound deliveries, internal warehouse movements (transfers, transports, internal sales, etc.) and batches already start with the goods receipt process and the determination of the correct goods receipt zone for receiving the products. A current example are the temperature-critical COVID-19 vaccines. It is essential that these products arrive in an adequately temperature-controlled goods receiving area. Such control can be achieved via the warehouse product master data. By using data loggers, it is easy to track in the incoming goods process whether the cooling chain was not broken during transport.
Safety and quality are top priorities in the pharmaceutical sector
Especially the quality inspection process is of great importance in the pharmaceutical sector. From the SAP EWM point of view, it is an option to map the quality inspection directly in SAP EWM via the Quality Inspection Engine, via an integration to quality management in SAP ERP or any external quality management system.
The materials can be sampled in an internal laboratory or samples can be sent to an external laboratory and the remaining stock is collected as quality inspection stock in the warehouse. As soon as the sample report for the material is available, the material is then posted to either unrestricted-use stock or restricted stock.
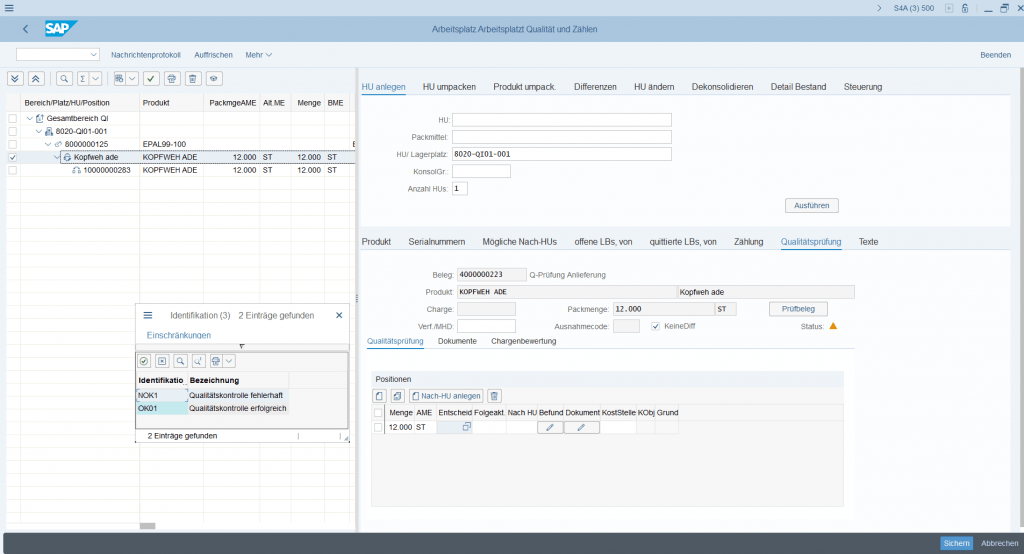
It is important that the respective product/batch combinations may only be delivered to the customer if the associated certificates permit approval for them.
Frequently, the same question arise as to which group of people is allowed to handle critical products. In this case, it is important to create an appropriate authorization concept at an early stage so that only trained personnel are allowed to handle narcotics, for example.
It is also important to ensure the highest level of safety and quality in the outgoing goods process. In order to avoid incorrect picking in the picking process, not only the handling unit and/or the storage location from which the pick is made is scanned during picking, but also the FMD barcode (“Falsified Medicines Directive” – EU counterfeiting directive) of the unit being picked. This provides additional security in the picking process by means of further verification.
In order not to lose speed and efficiency in the process, a multiscan can be used with the appropriate hardware, for instance. In this case, several barcodes are scanned by a camera and reported back to the system. Parallel picking of around ten folding cartons is thus no problem.
Unique challenges for intralogistics
Alongside inbound and outbound processes, internal warehouse processes are also of particular importance, especially in the case of a 3PL (Third Party Logistics). For example, individual cartons are combined into multipacks, products are labeled, or displays are built and stocked. Another challenge is that the same product from several suppliers can sometimes be present in the warehouse and must be properly managed by the system.
It is also necessary to comply with certain specifications when storing raw materials. Certain raw materials must not be stored on top of or next to other products because damage can affect the other products, even if they are packaged. A wide variety of packaging materials are used in the pharmaceutical environment, ranging from cardboard boxes, pallets, and drums to silos. An optimal storage strategy must be formed from the above points and the specific warehouse layout.
Narcotics products are special in several aspects. For example, they may be stored in a high rack, but only in the center – at the edge or on the lower levels they could be stolen too easily. The labels must also meet certain requirements, and the narcotics delivery note for further transport of the goods to the customer is mandatory. In addition, special training and authorizations are required for staff to handle narcotics.
The following applies to all products: Traceability must be ensured at all times; no goods movement or transfer posting takes place without system support. The verification of handling units or storage locations is carried out simply by scanning, for example, via the RF or FIORI applications in the SAP standard. Furthermore, with the warehouse management monitor, the SAP EWM standard offers a powerful tool for seamless tracking of all activities in the warehouse.
Production supply and disposal is of central importance in the pharmaceutical industry
For manufacturing pharmaceutical companies, a key issue is production integration. This involves supplying production with raw materials and transferring the finished products from production to the warehouse. Here, the raw materials that need to be supplied can be reported to SAP EWM, for example, via a process order based on a master recipe. During the physical provision of raw materials, a scale interface can be integrated, among other things. Thus, a difference in weight between incoming weighing in production and outgoing weighing on return from production can result in a quantity to be posted.
Another possibility to post the correct quantities in real time is the connection of a production management system via MES interface. The quantities consumed are reported via interface from the production management system to SAP EWM, where they are posted directly by the associated handling unit.
For the production disposal of finished products, the connection of a pharma-specific software for the determination of serial numbers can be used. This software reports the serial numbers for the products to SAP EWM, where they are stored and printed on the folding boxes.
Pharmaceutical products: great importance - great requirements
The Corona pandemic has shown the general public how important logistical processes around pharmaceutical goods are. It is important to understand that the requirements for a warehouse management system in the pharmaceutical industry are extremely complex. However, SAP EWM offers many key solution components out-of-the-box, such as the Quality Inspection Engine, Warehouse Management Monitor, Batch Management, restrictions on storage, and an interface into SAP EH&S Dangerous Goods Management.
We are here for you
We support you in adapting your warehouse processes to the specific needs of your industry and in making your operational processes more efficient, transparent and cost-saving. Have we aroused your interest? Then please do not hesitate to contact us. If you have any questions about this or other topics in the blog, please contact blog@leogistics.com.
Florian Schorcht
Consultant SAP Logistics



